38+ adiabatic compression work calculator
D actual diameter of pipe in inches. τ hr - ha - Q a r 412 412 REFERENCE COMPRESSION.

Atomic Scale Carving Of Nanopores Into A Van Der Waals Heterostructure With Slow Highly Charged Ions Acs Nano
The system can be considered to be perfectly insulated.
. D the volume of compressed air in cubic feet per minute discharged at the final pressure. 1 shows a gas confined by a membrane to one side of a two-compartment thermally insulated container. P is kW H ad Q m 1000 With.
Web It consists of two adiabatic and two isothermal processes. Web This assumes that the expansion takes place adiabatically and reversibly and that the change in enthalpy of the stream is counterbalanced by the change in kinetic energy of the stream. Web including the compression work.
And Q 0 for an adiabatic process. HP 144 N P1 V k 33000 k - 1 P2 P1k - 1 N k - 1 1 where. In a gasoline engine the pressure inside the piston changes when the fuel is combusted inside the piston releasing thermal energy and causing the volume inside the piston to increase.
Web 38 adiabatic compression work calculator Senin 20 Februari 2023 Adiabatic Processes Adiabatic Compressor Review Youtube The Charge Exchange Of Slow Highly Charged Ions At Surfaces Unraveled With Freestanding 2d Materials Sciencedirect Baca Juga 30 The Remarried Empress Chapter 15 15 Venus 2000 Review 26 God Of. Web This calculator performs thermodynamic calculations for adiabatic expansion of an Inner ideal gas against an Outer ideal gas separated by a massless frictionless piston. Since we are treating the vapor as an ideal diatomic gas we can use γ 7 5 14.
For the adiabatic reversible expansion the change in entropy per unit mass of the flowing stream is zero. The gas in the left chamber expands freely into the right chamber when the membrane is punctured. Web This schematic also shows how the movement of the piston can be translated into other motions within the engine.
Adiabatic compression assumes perfect insulation which is purely theoretical. Web Figure 37. So after the compression the pressure of the mixture is p 2 100 10 5 Nm 2 240 10 6 m 3 40 10 6 m 3 140 123 10 6 Nm 2.
In an adiabatic process energy is transferred only as work. The surroundings continue to work on the working substance compressing it further. Im thinking about how to derive an expression for the final temperature call it T2 and the work done call it W in an adiabatic compression when you only know the initial and final pressures call them P1 and P2 and the initial temperature call it T1.
Web Adiabatic compression work W can be calculated from equn1 or equn2 since ΔU Q W. Web For an adiabatic compression we have p 2 p 1 V 1 V 2 γ. And this is the equation we use to calculate the amount of work done in compressing the gas.
Result JK JK J where ΔW work done by the system n number of moles R universal gas constant T initial temperature T f final temperature C p specific heat at constant pressure. Im not sure about my expression though. CVinnerdTinner - PoperatingdVinner CVouterdTouter - PoperatingdVouter.
C a coefficient varying with the diameter of the pipe as determined by experiment. Another interesting adiabatic process is the free expansion of a gas. Hr - ha ΔK τ Q a r 411 Assuming that the fluid velocities are low which is legitimate if we consider state in the discharge tank we get.
Assuming the process a-r is known the compression work τ is given by 236 which is written here. At the end of Step 4 the working substance is precisely in the same state as before Step 1. Since the temperature of the system is returned to its original state the internal energy change of the total process is zero.
The compression results in an increase in temperature to T_text h T h. This equation applies to both irreversible and reversible expansions. W P d V.
N number of compression stages. These are numerical integrations of the relationships. The calculation can be a bit involved because as we compress the gas the pressure will change ie.
Web A d x d V so. In a reversible expansion the pressure of the gas is uniform throughout and is not affected by the rate at which the gas is deforming since the rate is negligible. Web The work required during the compression is equal to the adiabatic head multiplied by the mass flow rate of gas and divided by 1000 in order to express it in kW.
Web The power required for adiabatic process without transfer of heat compression of air can be expressed as. W 8103 x 101325 x 10 5 x 24616 - 101325 x 10 5 x 12308 03 257985 MJ. P is Power kW H ad Adiabatic head Nmkg Q m Compressor throughput kgs 1000 WkW.
This engine absorbs heat from a hot reservoir transforms it into work and releases the rest of the heat to the cold one. Web Adiabatic compression. The pressure is a function of volume P V.
Web Adiabatic compression is a process where there the PV work done is negative and it results in increase temperature of system. This rise in temperature increases the internal energy of the system. With this combined gas law calculator you can design any kind of thermodynamic cycle and find out how this change influences output efficiency.
Web An adiabatic process is a thermodynamic process in which there is no heat transfer into or out of the system Q 0. Web Calculating work in Adiabatic compressions. Web This formula is.
Web Work Done in Adiabatic Process Calculator This Calctown Calculator calculates the work done by the system in an Adiabatic Process.

1st Puc Kseeb Solutions

Thermodynamics Isentropic Process Ideal Gases Reversible Work Isentropic Eff 21 Of 25 Youtube
Adiabatic Equation Calculator Apps On Google Play

Resolucao Himmelblau 7 Ed Pdf
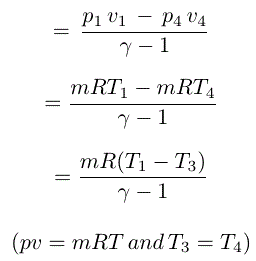
4 Stages Of Carnot Cycle Improving Thermal Efficiency Mechanicaltutorial

1st Puc Kseeb Solutions
Adiabatic Equation Calculator Apps On Google Play

Chapter 2 The First Law Unit 3 Adiabatic Process Ppt Download

Calculate Workdone In Adiabatic Compression Of One Mole Of An Ideal Gas Monoatomic From An Initial Pressure Of 1 Atm To Final Pressure Of 2 Atm Initial Temperature 300 K If Process

In A Closed System Is The Work Done By The Compressor Minimum In An Adiabatic Process And Maximum In Isothermal Quora

Adiabatic Compression Of An Ideal Gas Youtube
Calculate Work Done In Adiabatic Compression Of One Mole Of An Ideal Gas Monoatomic From An Initial Pressure Of 1atm Sarthaks Econnect Largest Online Education Community
Adiabatic Equation Calculator Apps On Google Play

Calculate Workdone In Adiabatic Compression Of One Mole Of An Ideal Gas Monoatomic From An Initial Pressure Of 1 Atm To Final Pressure Of 2 Atm Initial Temperature 300 K If Process

Pdf Atomic Scale Carving Of Nanopores Into A Van Der Waals Heterostructure With Slow Highly Charged Ions
Essd Global Climate Related Predictors At Kilometer Resolution For The Past And Future
Structural And Electronic Properties Of Various Useful Metal Oxides Springerlink